|
LATEST NEWS: Epitracker uncovers groundbreaking science that may help delay aging in dolphins and humans. Read our press release and the PNAS paper.
|
At Epitracker, we are committed to dolphin health.
Epitracker and its founders have been committed to understanding and improving dolphin health for 20 years.
2001. Dr. Stephanie Venn-Watson, Epitracker's co-founder and veterinary epidemiologist, departs from the Centers for Disease Control and Prevention to help track and understand marine mammal health and disease at the U.S. Navy Marine Mammal Program. Her work with the Navy results in 70 peer-reviewed scientific publications providing critical insight into dolphin health, be it dolphins at the U.S. Navy or in the wild.
2005. Dr. Venn-Watson co-founds the National Marine Mammal Foundation, a non-profit organization recognized globally as a leader in marine mammal science, medicine, and conservation.
2008. Drs. Stephanie and Eric Venn-Watson co-found Epitracker as a data analytics company that tracks diseases to find cures, including in dolphins.
2010. Epitracker helps to lead the U.S. government's investigation into the potential effects of the Deepwater Horizon oil spill on the health of wild dolphins living in the Gulf of Mexico. Outcomes of this investigation, including three key papers co-led by Dr. Venn-Watson, results in the largest settlement from an oil company to the U.S. government for an off-shore oil spill.
2011. Dr. Venn-Watson is appointed Chair of the Working Group for Marine Mammal Unusual Mortality Events by the Secretary of the Department of Commerce. This Working Group is a consortium of scientists who help to guide investigations of unusual marine mammal mortality events. She serves this role through 2014.
2015. Dr. Venn-Watson discovers a fish-based fatty acid that may help to attenuate metabolic syndrome (pre-diabetes) in dolphins, and perhaps humans, too.
2017. Epitracker establishes a Cooperative Research and Development Agreement with the U.S. Navy Marine Mammal Program to discover and develop novel therapeutics for aging-associated conditions in dolphins, primarily by analyzing archived Navy dolphin data and samples. Read more about how we leverage archived dolphin health data and samples to make ground-breaking discoveries to improve dolphin health.
2020. Epitracker's discovery of C15:0 as an essential fatty acid for dolphins and humans is published across two scientific papers in PLOS ONE and Scientific Reports. Epitracker helps to secure $6.2M Series A financing for its first spinout company, Seraphina Therapeutics, to advance C15:0 as a dietary supplement and food ingredient, including for dolphins.
2001. Dr. Stephanie Venn-Watson, Epitracker's co-founder and veterinary epidemiologist, departs from the Centers for Disease Control and Prevention to help track and understand marine mammal health and disease at the U.S. Navy Marine Mammal Program. Her work with the Navy results in 70 peer-reviewed scientific publications providing critical insight into dolphin health, be it dolphins at the U.S. Navy or in the wild.
2005. Dr. Venn-Watson co-founds the National Marine Mammal Foundation, a non-profit organization recognized globally as a leader in marine mammal science, medicine, and conservation.
2008. Drs. Stephanie and Eric Venn-Watson co-found Epitracker as a data analytics company that tracks diseases to find cures, including in dolphins.
2010. Epitracker helps to lead the U.S. government's investigation into the potential effects of the Deepwater Horizon oil spill on the health of wild dolphins living in the Gulf of Mexico. Outcomes of this investigation, including three key papers co-led by Dr. Venn-Watson, results in the largest settlement from an oil company to the U.S. government for an off-shore oil spill.
2011. Dr. Venn-Watson is appointed Chair of the Working Group for Marine Mammal Unusual Mortality Events by the Secretary of the Department of Commerce. This Working Group is a consortium of scientists who help to guide investigations of unusual marine mammal mortality events. She serves this role through 2014.
2015. Dr. Venn-Watson discovers a fish-based fatty acid that may help to attenuate metabolic syndrome (pre-diabetes) in dolphins, and perhaps humans, too.
2017. Epitracker establishes a Cooperative Research and Development Agreement with the U.S. Navy Marine Mammal Program to discover and develop novel therapeutics for aging-associated conditions in dolphins, primarily by analyzing archived Navy dolphin data and samples. Read more about how we leverage archived dolphin health data and samples to make ground-breaking discoveries to improve dolphin health.
2020. Epitracker's discovery of C15:0 as an essential fatty acid for dolphins and humans is published across two scientific papers in PLOS ONE and Scientific Reports. Epitracker helps to secure $6.2M Series A financing for its first spinout company, Seraphina Therapeutics, to advance C15:0 as a dietary supplement and food ingredient, including for dolphins.
Relevance of dolphins to human health (and how we can help dolphins, too)
Ends up, bottlenose dolphins and humans have a lot in common. Here, we share how dolphins and humans are similar, and why helping one species can also help the other.
Evolutionary History of Dolphins
Bottlenose dolphins (Tursiops truncatus) are part of the cetacean clade, which includes whales, dolphins, and porpoises. Approximately 53 million years ago, ancestors of dolphins and other cetaceans transitioned from living on land to living in the marine environment. As members of the order Artiodactyla, dolphins and other cetaceans are most closely related to cattle, pigs, and camels. Despite their distant evolutionary relationship to humans, dolphins and humans have unexpected and important similarities related to longevity and aging-associated diseases, including large brain sizes, rapid glucose transport systems, conserved chromosomes, and co-evolved metabolomes and gene regulation to support long lives. Each of these parallels is described in more detail below.
Large Brain Size & Rapid Glucose Transport Systems

Only two groups of animals in the world, primates and cetaceans, have coincidentally evolved over tens of millions of years to develop increased brain size with static or decreased body size. Interestingly, among primates and cetaceans, the two species with the highest brain-to-body size ratio (also called the encephalization quotient, or EQ), are humans first and bottlenose dolphins second.
High EQs may be drivers for co-evolved glucose transport systems that enable red blood cells to rapidly carry glucose to the brain and other tissues. While it was once believed that only primates sustained this GLUT-1 mediated transport system from the neonatal period into adulthood, this same system was discovered in cetaceans, including bottlenose dolphins. Craik et al. (2008) summarized that there is ‘…remarkable functional similarity in the glucose transport properties of red blood cells from adult dolphins and humans.’ These similarities may, in part, help to explain why dolphins and humans are susceptible to similar glucose-associated diseases, including chronic hyperglycemia, prediabetes, and metabolic syndrome.
High EQs may be drivers for co-evolved glucose transport systems that enable red blood cells to rapidly carry glucose to the brain and other tissues. While it was once believed that only primates sustained this GLUT-1 mediated transport system from the neonatal period into adulthood, this same system was discovered in cetaceans, including bottlenose dolphins. Craik et al. (2008) summarized that there is ‘…remarkable functional similarity in the glucose transport properties of red blood cells from adult dolphins and humans.’ These similarities may, in part, help to explain why dolphins and humans are susceptible to similar glucose-associated diseases, including chronic hyperglycemia, prediabetes, and metabolic syndrome.
Conserved Chromosome 1
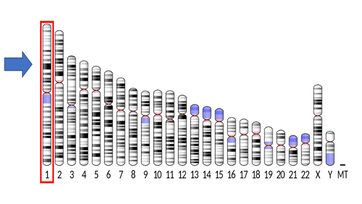
Human chromosome 1 (hsa1) is the largest of human chromosomes, containing 249 million base pairs and 300 genes. Hsa1 is an ancient chromosome that has been entirely conserved in very few other taxa, including great apes and bottlenose dolphins (Murphy et al. 2003). While faster evolutionary rates of other species appear to have resulted in changes to chromosome 1, the relatively slower evolutionary rates of humans, great apes, and dolphins likely explain why these species have conserved hsa1, resulting in the observed ‘…extraordinary degree of conservation of genome organization [that] has occurred since the divergence of lineages leading to man and dolphin.’
In humans, hsa1 represents 8% of DNA in cells and more than 148 disease genes, including those related to Alzheimer’s disease, hemochromatosis, and hypercholesterolemia. Similar to humans, dolphins are susceptible to developing these diseases, especially with increased age.
In humans, hsa1 represents 8% of DNA in cells and more than 148 disease genes, including those related to Alzheimer’s disease, hemochromatosis, and hypercholesterolemia. Similar to humans, dolphins are susceptible to developing these diseases, especially with increased age.
Co-Evolved Metabolomes and Gene Regulation to Support Longevity
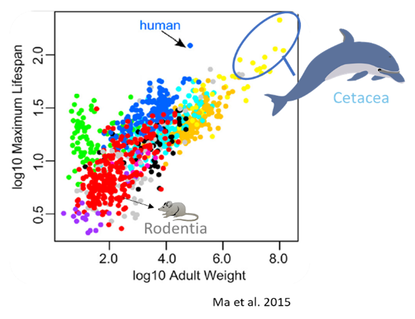
When assessing ratios of maximum lifespan-to-adult weight, humans are an outlier among primates, having longer than expected lifespans. The mammalian clade with the longest lifespans are cetaceans.
There are recognized differences in molecular phenotypes when comparing long-lived and short-lived species. This includes distinct amino acid and lipid profiles in the metabolome, proteins and genes related to DNA repair, and lipid components of cell membranes. Not surprisingly, these adaptations appear to help long-lived species resist environmental stressors better than short-lived species, thus enabling them to live longer. As such, studying metabolomes, gene expression, and cell membranes in long-lived species, including dolphins, can provide novel insight into how to expand longevity.
There are recognized differences in molecular phenotypes when comparing long-lived and short-lived species. This includes distinct amino acid and lipid profiles in the metabolome, proteins and genes related to DNA repair, and lipid components of cell membranes. Not surprisingly, these adaptations appear to help long-lived species resist environmental stressors better than short-lived species, thus enabling them to live longer. As such, studying metabolomes, gene expression, and cell membranes in long-lived species, including dolphins, can provide novel insight into how to expand longevity.
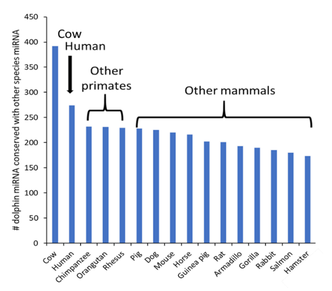
Our in-house studies support strong conservation of metabolomic and microRNA profiles between dolphins and humans. Metabolomics involves the study and measurement of thousands of small molecules present in the body. MicroRNA are small, noncoding RNA that can post-transcriptionally impact gene expression.
One hundred metabolites present in dolphin serum were found to be predictive of healthier metabolic states. Of these 100 molecules, 83% are known or assumed to be present in human serum. When assessing dolphin microRNA, 400 known microRNA were present in dolphin serum. Of these, 275 (69%) were conserved in humans. Interestingly, four primates (humans, chimpanzee, orangutan and rhesus) were among the top five species with the highest number of conserved microRNA in dolphins. These similarities support the relevance to humans of our discoveries in dolphin metabolomes and microRNA.
One hundred metabolites present in dolphin serum were found to be predictive of healthier metabolic states. Of these 100 molecules, 83% are known or assumed to be present in human serum. When assessing dolphin microRNA, 400 known microRNA were present in dolphin serum. Of these, 275 (69%) were conserved in humans. Interestingly, four primates (humans, chimpanzee, orangutan and rhesus) were among the top five species with the highest number of conserved microRNA in dolphins. These similarities support the relevance to humans of our discoveries in dolphin metabolomes and microRNA.
Similar Aging-Associated Diseases
Similar genetic, molecular and cellular profiles between humans and dolphins, as well as their shared long lives, likely explain why humans and dolphins develop similar chronic aging-associated diseases. As dolphins age from 30 to 50 years old, they are more likely to develop chronic inflammation and dyslipidemia. Like humans, dolphins are susceptible to metabolic syndrome, including elevated insulin, glucose, cholesterol, triglycerides, and liver enzymes. The dolphin phenotype of metabolic syndrome also includes dysmetabolic iron overload syndrome, fatty liver disease, and nonalcoholic steatohepatitis, also called NASH. Further, older dolphins can develop central neurodegenerative diseases, including development of amyloid-beta plaques and phosphorylated-tau tangles that parallel lesions present in people with Alzheimer’s disease. Based on their findings, Gunn-Moore et al. concluded that, “Dolphin, like man, an animal with exceptional longevity, might be one of very few natural models of Alzheimer’s disease”. All of these conditions have been documented in wild dolphins, supporting a wild-type susceptibility to chronic, aging-associated diseases.
The many parallels between dolphins and humans support that discoveries made to improve dolphin health may also improve human health, and (just as importantly) vice versa. Additionally, we are able to apply these discoveries to help understand wild dolphin health and how changing oceans and prey availability may impact conservation efforts for wild dolphins.
The many parallels between dolphins and humans support that discoveries made to improve dolphin health may also improve human health, and (just as importantly) vice versa. Additionally, we are able to apply these discoveries to help understand wild dolphin health and how changing oceans and prey availability may impact conservation efforts for wild dolphins.
